Battery Research
概览
Shedding Light on the Workings of Energy Storage Materials
Energy generation and energy storage related applications require some of today’s most complex materials development initiatives to meet efficiency and reliability targets. Many of our electronic devices, from laptops to smartphones, are powered by rechargeable lithium-ion (Li-ion) batteries, and they could soon extend into many other areas as well. This includes transport, through the ongoing development and adoption of electric vehicles. New materials are continuously being developed that transform the ways we capture, transmit, and store energy.
The performance of any battery, whether in terms of its capacity, lifetime or energy density, is ultimately down to the intrinsic properties of the materials that comprise its anode, cathode, electrolyte and SEI. Bruker has developed a comprehensive suite of characterization techniques to enable scientist to understand and optimize the physical and chemical properties, performance and stability of all battery components and the fully assembled battery cells.
Read on to find out how Atomic Force Microscopy, FTIR Spectroscopy, Nanomechanical Testing, X-ray Diffraction, Raman Microscopy, X-ray Microscopy, and X-ray Spectroscopy shed light on the workings of energy storage materials.
In-situ Characterization
In-situ Characterization
Investigating solutes and electrodes
Researchers can in-situ monitor the electrochemical process in the solutes and electrodes of a lab-level battery model system. These model systems are not ready battery products, but one has the possibility to tune the anode, cathode materials, the electrolyte composition, temperature etc. during a programmed voltage cycle. FTIR spectroscopy is synchronised with electrochemical reaction. As result IR spectra over time / potential are collected. The combination of FTIR spectroscopy with electrochemistry offers insight in the molecular change and the reaction process of the studied molecules in addition to the electrochemical response of the experiment.
Follow battery cell behavior during cycling
During charge/ discharge, the cathode and anode of every battery cell undergo constant changes, e.g. due to the insertion of Li-cations. With X-ray diffraction (XRD), both the changing phase composition and the evolution of the crystal structure can be followed simultaneously. This allows researchers to understand new energy storage materials on an atomic level, follow the reaction that occur during cycling and monitor degradation behaviour to improve battery performance.
Our X-ray diffractometers support your research and development in battery materials, from ex-situ analysis of isolated cathode and anode materials, to the in-operando investigation of fully functional coin- and pouch-cells.
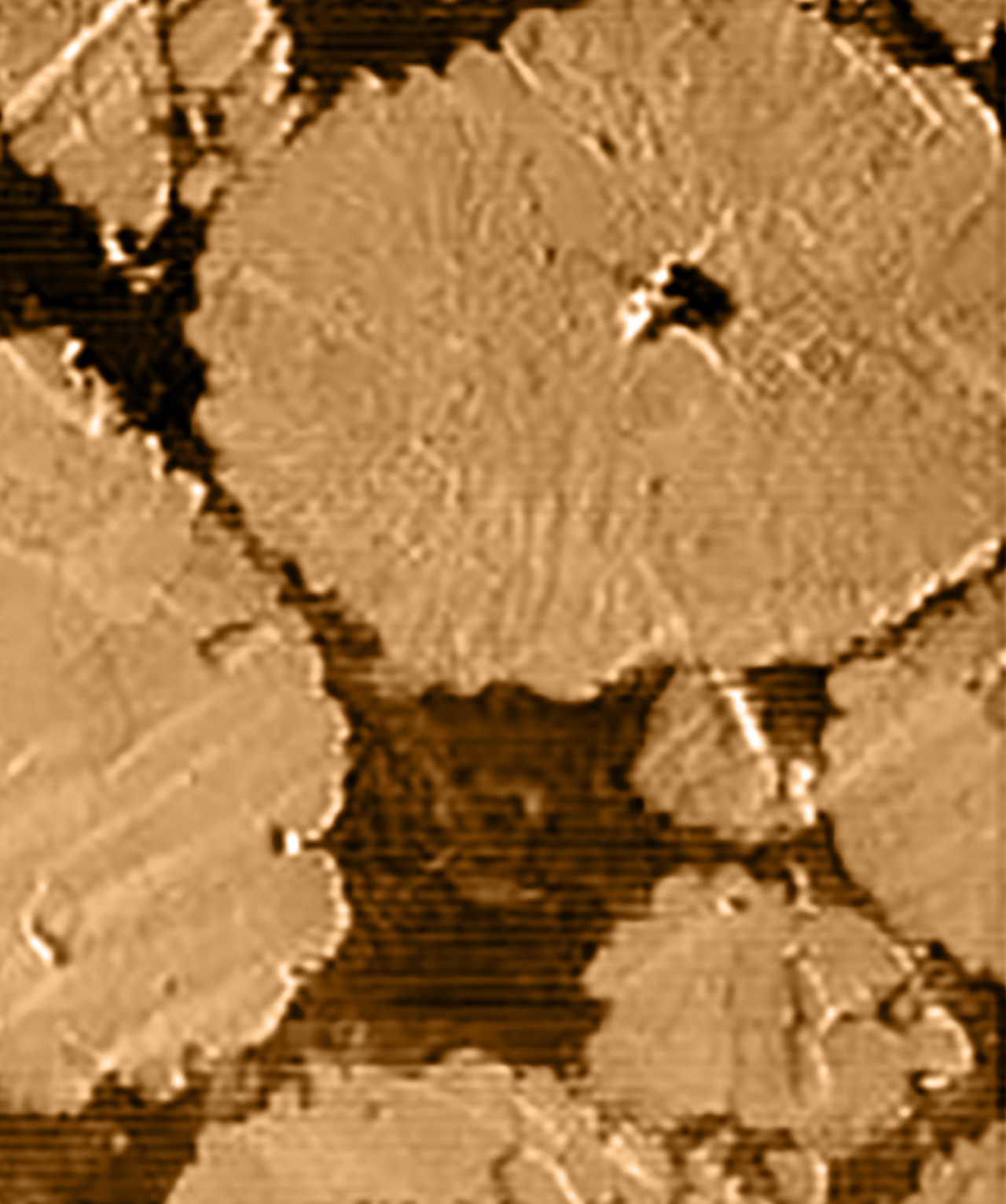
Observing Li-dendrite growth in situ
Lithium dendrite growth is one of the biggest problems affecting the safety of Li-ion batteries, but probing the initial stages of dendrite growth is difficult due to the reactive and fragile nature of lithium compounds, especially when studying growth at the solid electrolyte interface (SEI).
使用atomic force microscopy with electrochemical mode, the morphological evolution of the electrode surface under potential control can be traced. These experiments reveal different Li-deposition on graphite for different electrolytes, providing a deeper understanding of the underlying mechanism of dendritic growth in Li-batteries.
Ex-situ and Failure Analysis
Ex-situ and Failure Analysis
Studying electrochemical side reactions by laser desorption/ionization imaging
In the emerging fields of electroorganic synthesis and battery research, electrochemical side reactions on the active surface of electrodes represent a major challenge for efficiency and reproducibility.
Often, the undesired polymerization of one or more compounds on the active surface of electrodes is observed. These polymers tend to adsorb on the electrode leading to a passivation of the active surface, which is often referred to as “electrode fouling”.
Mass spectrometric imaging using the timsTOF fleX enables the identification and the spatially resolved visualization of the adsorbed side products. Hence, timsTOF fleX-based imaging allows the investigation of electrode fouling and provides valuable insight into electrochemical reaction pathways.
Increasing battery safety
Mechanical damage, including brittle failure of the electrodes and separator penetration, can give rise to dramatic releases of stored energy, including battery fires. Moreover, failures of coatings, mechanical (or ion) induced swelling and stiffening, stresses arising from fabrication, and mechanical stresses and damage from multiple charge-discharge cycles pose significant challenges for new device development and integration. Thus, for both safety and performance reasons, it is necessary to understand how these devices perform mechanically, including each component at the appropriate size scale.
电池材料的纳米机械测试提供雷竞技网页版es quantitative characterization for emerging materials and deeper insight for improving mechanical performance.
Carbon analysis in flexible electrodes
Batteries using LiFePO4 (LFP) based cathodes are known to be very safe and show no risk of thermal runaway but have a low electrical conductivity, limiting the performance at high charge/ discharge rates. A very thin carbon coating on the LFP particles can improve its conductivity. The anodic stability of carbon coated cathode materials can be studied with Raman Spectroscopy, which demonstrates the homogeneity of the coating.
All components of a battery like anode/cathode materials and electrolytes can be analyzed with a very high lateral resolution using Raman microspectroscopy, both ex- and in -situ. Carbon is widely used in batteries. Raman spectra can be used to distinguish its allotropes and provide further information like defect concentration.
Verify structural integrity and research microstructure of electrodes
X-ray microscopy enables to non-destructively visualize the internal 3D structure of batteries and fuel cells. XRM is therefore a great tool to help understanding failure mechanisms by monitoring the internal alignment of components such as electrode separation over the battery life time, or in stress tests.
The electrode microstructure of modern high-performance batteries such as Li-ion batteries significantly impacts key properties such as cycle life time and capacity. A lot of efforts therefore go into careful optimization of processing parameters to tease out the best battery performance. XRM as multi-scale analysis technique supports advanced battery research since it can reveal at high resolution the microstructure of the individual anode and cathode layers.
Elemental mapping in lead-acid battery electrodes
Lead-acid batteries (accumulators) are rechargeable devices for storing electric energy generated by electrochemical processes. The batteries consist of electrodes made of lead (Pb) and lead dioxide (PbO2) and dilute sulfuric acid (37% H2SO4) as electrolyte. During discharge of lead-acid batteries, finely dispersed lead sulfate (PbSO4) forms on electrodes in a process that is reversed by recharging. However, under certain conditions, permanent deposits can also form on the electrodes. X-ray element maps acquired by WDS are ideal for investigating the nature and spatial distribution of sulfation deposits leading to battery failure.